Enzymes are specific organic molecules found in biological systems
Enzymes are specific organic molecules found in biological systems that allow cellular life to exist and function at earth temperatures. Most life-supporting chemical reactions could only occur above 90°C or 200°F in the absence of enzymes.[1] Enzymes are referred to as macromolecular biological catalysts. They allow the existence of reactions that would not occur otherwise under numerous conditions having to do with temperature, pH and atmospheric conditions within the human body. Metabolic processes within cells require enzyme catalysts in order to occur at rates fast enough to support life. Enzymes are known to catalyze more than 5,000 biochemical reaction types.[2] The study of this complex topic is called enzymology. Enzymes accelerate the chemical reaction rate in numerous ways, lowering the activation energy. Activation energy is the energy that must be available to a chemical or nuclear system with potential reactants to produce a reaction or product. Enzymes react with other substances, either to take them apart or join them together.[3] They do not alter the position of the chemical equilibrium of the reaction.
Enzymes accelerate the chemical reaction rate in numerous ways, lowering the activation energy. Activation energy is the energy that must be available to a chemical or nuclear system with potential reactants to produce a reaction or product. Enzymes react with other substances, either to take them apart or join them together.[3] They do not alter the position of the chemical equilibrium of the reaction.
During the presence of an enzyme, the reaction moves in the same direction as it would without the enzyme, however enzyme presence accelerates the process.
Function and Nature of Enzymes
Enzymes are responsible for:
- Signal transduction and cell regulation are often by kinases and phosphatases.
- Generating movement with myosin (muscle protein) hydrolyzing ATP to generate a muscle contraction.
- Transporting cargo around the cell as part of the cytoskeleton.
- Digestion, metabolism, respiration
- Digestive enzymes such as amylases and protease break down large molecules of starches or proteins into smaller ones for proper absorption in the intestines.
- Hormone production.
- Nutrient absorption and transportation.
- Cellular repair and division.
- Detoxification
Disease: Viruses can contain enzymes for infecting cells, i.e. HIV integrase and reverse transcriptase.
Enzymes
- Stabilize the transition state: a. Create an environment with a charge distribution complementary to that of the transition state to lower its energy.
- Provide an alternative reaction pathway: a. Temporarily reacts with the substrate, forming a covalent intermediate to provide a lower energy transition state.
- Destabilizes the substrate ground state: a. Distort bound subtract(s) into their transition state form to reduce the energy required to reach the transition state. b. Orient the substrates into a productive arrangement to reduce the reaction entropy (thermodynamic) change.
Enzymes essentially react with other substances, either to take them apart or join them together.
Enzymes are divided into [4] :
- Simple: contains the protein part only (e.g., hydrolases like pepsin, trypsin or ribonuclease).
- Complex: Proteins may be joined with a non-protein part, referred to as prosthetic groups. The protein part is called the apoenzyme. The non-protein part is referred to as a Cofactor. Together, apoenzyme and Cofactor, form a biologically active molecule of enzyme – the holoenzyme.
Cofactors:
- Metal ion: helps the enzyme position the substrate molecule into the active site. Called activators, the associated metals may include copper, cobalt, zinc, magnesium, molybdenum and manganese.
- Organic molecule: often vitamins such as riboflavin, the B vitamins and vitamin C.
- Coenzymes: a non-protein organic molecule that binds to the molecule of apoenzyme freely, and thus can detach from it, i.e. NAD+ (Nicotinamide adenine dinucleotide) and NADP+ (Nicotinamide adenine dinucleotide phosphate). NAD+ and NADP+ are electron carriers in cellular respiration. NADP+ is created in an anabolic reaction, or a reaction that builds large molecules from small molecules.
- Prosthetic group: a non-protein organic molecule that binds to the molecule of apoenzyme tightly, i.e. heme, FAD (flavin adenine dinucleotide)
A substrate
The molecules upon which enzymes react is called a substrate. The enzyme remains intact and is not consumed during chemical reactions. Nor do they alter the stability of a reaction. Instead, they support the progression of a reaction maintaining equilibrium. The majority of enzymes are proteins made up of amino acids, the basic building blocks within the body.
There are exceptions with some kinds of RNA molecules called ribozymes.[5] Amino acid molecules are connected through linkages known as peptide bonds that form proteins.
Enzymes are made up of a different number of peptide chains and are termed multienzyme complexes. An example of multienzyme complexes would be the fatty acid synthase, an enzyme catalyzing the synthesis of higher fatty acids in cells.[6] Chemically, the small groups of bonded amino acids are called polymeric molecules or referred to in biochemistry as polypeptides.
It takes 30 amino acids to form a long enough chain that enables the molecules to influence their own shape in becoming a protein. Whether one chain or multiple chains, it contains multiple parts called domains. Proteins serve numerous biochemical functions including anatomical structural features in organisms, nutrient carriers, antigens and hormones.[7]
The Enzyme – Substrate Complex
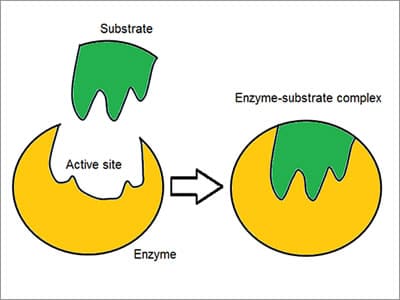
Coined as a “Lock and key” model, enzymes react with other substances to either take them apart or join them together. [8]There is a high specificity of enzymes demonstrated by the way they associate or bind with the substrate: the reactant molecule. Chemoselective, every enzyme molecule has an active site on its surface. The reactant molecule is attracted to and moulded into the indentation that forms the active site.
Specificity is achieved by binding pockets with complementary, shape, charge and hydrophilic/hydrophobic characteristics to the substrates. The active site continues to change until the substrate is completely bound, resulting in a final shape.
That said, enzymes are flexible structures and the active site is continuously reshaped by interaction with the substrate as it interacts with the enzyme.
Controlling Enzyme Activity
Enzymes guide and regulate the metabolism of a cell and are carefully controlled.[9] The mechanism of action of enzymes involves regulatory molecules that can either increase (activator) or inhibit (inhibitors) the activity of an enzyme. An enzyme inhibitor is a molecule that binds to an enzyme and blocks the binding of a substrate, decreasing its activity. If an enzyme produces too much of a substance in an organism, that substance begins to act as an inhibitor for the enzyme at the beginning of the pathway as a form of negative feedback, slowing the reaction down. Drugs can be enzyme inhibitors. For example, blocking an enzyme’s activity can kill a pathogen or correct a metabolic imbalance.[10]
The control of enzyme activity is essential for homeostasis in the body. When there is a malfunction of an enzyme such as a mutation, over or under production, or deletion, this can lead to a genetic disease. In some cases, it can be fatal. For example, pancreatic insufficiency is a condition that occurs when the pancreas does not make enough of a specific enzyme required to digest food in the small intestine.[11]
Table 1 - Factors affecting or controlling enzyme activity [12]

Temperature modulation
The catalytic activity of enzymes requires optimum temperature within the body. Human enzymes have maximal activity at 37oC. Enzymes can become vulnerable to temperature changes. Due to their protein nature, applying high temperatures between 55-60o C causes denaturing of protein, producing a conformational change and destruction of protein. This change causes a drop-in or a complete halt of the reaction.[13] Moreover, low temperatures can slow reactions, reducing the activity of enzymes.
pH
Enzymes are sensitive to changes in pH. As in temperature changes, extremes of low and high again lead to denaturation of the molecules. The concentration of H+ affects the ionization of acidic and base groups
Enzyme Sources [14]
- Metabolic enzymes: regulates organs, tissue, and blood. Help create new cells, repair existing damaged cells and move nutrients to where your body most needs them.
- Digestive Enzymes: breaks down food. Subtypes – amylase, lipase, protease
- Raw foods: Supports the immune system, and cellular repair.
There are six major classes of enzymes with specific functions. There are also subgroups.
Table 2 – Enzyme types [15,16]
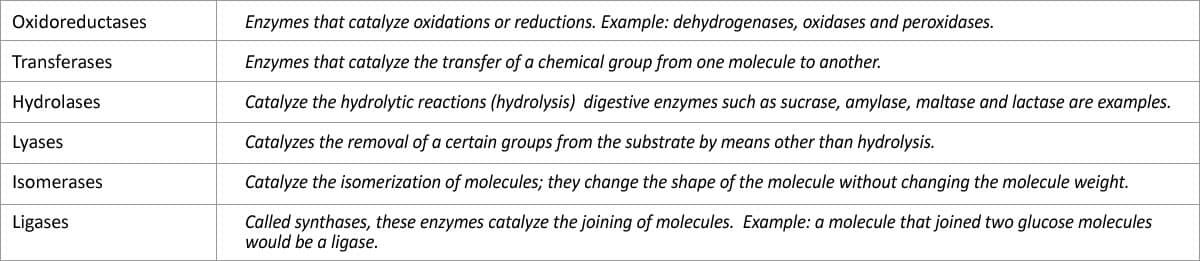
Enzyme subgroups:
Hydrolases are enzymes that split out water, separating the parts of molecules.
Hydrogenases are enzymes that add hydrogen atoms to other molecules; 5 alpha-reductase is an example.
Oxidases catalyze oxidations by adding oxygen or electrons to molecules or atoms.
Enzymes in the Skin; Building of the skin barrier and the final desquamation process
The entire epidermal differentiation process is dependent upon enzymatic activity. Lipid hydrolases are responsible for the conversion of lipids into ceramides and free fatty acids. Enzymes are involved in profilaggrin modification and proteolytic processing in the epidermis. [17] The healthy transitional phases of the entire building process of the stratum corneum are vital and dependent upon enzymes.
Protease enzymes are essential to the normal desquamation process within the cells of the stratum corneum (SC). Desmosomes are important for strong cell-to-cell adhesion. The desquamation process involves the proteolysis of intercellular adhesive structures created in the desmosome structures. There are specific protease enzymes within the stratum corneum called tryptic enzyme and chymotryptic enzyme involved with the degradation of the corneodesmosomes. Two proteins found in desmosomes are desmoglein and desmocollin, located at the interconnections within cells.
The stratum corneum chymotryptic enzyme is produced as an inactive precursor with no proteolytic activity. Hence there is a requirement for an activating enzyme that involves an enzyme with a trypsin-like substrate. Different proteases attack the different amino acid sites on the desmoglein and desmocollin proteins. When bonds are weakened, they break, allowing cells to flake off.
The desquamation process requires water from within the epidermis and a normal pH. The orchestration of all biological activities is complex and must work synergistically with one another.
References
1. Pugliese, Peter T. MD. (2005) Appendix A. Enzymes and Enzyme Activity. Advanced Professional Skin Care – Medical Edition. p 390-392 The Topical Agent, LLC, Burnsville, PA
2. Enzyme. Wikipedia.org Retrieved from https://en.wikipedia.org/wiki/Enzyme
3. IBID – Pugliese – Ref 1
4. IBID – Lavrikova p.
5. Subchapter Author: Petra Lavrikova. Chapter 6. Enzymes. Function of Cells and Human Body. Retrieved from http://fblt.cz/en/skripta/ii-premena-latek-a-energie-v-bunce/6-enzymy/
6. IBID – Lavrikova p.
7. IBID - Pugliese
8. IBID – see ref. 2
9. Enzyme Regulation. Biology Energy and Enzymes Retrieved from https://www.khanacademy.org/science/biology/energy-and-enzymes/enzyme-regulation/a/enzyme-regulation
10. Enzyme Inhibitor Retrieved from https://en.wikipedia.org/wiki/Enzyme_inhibitor
11. Pancreatic Insufficiency. Digestive Disease Center, Medical University of South Carolina. Retrieved from http://ddc.musc.edu/public/diseases/pancreas-biliary-system/pancreatic-insufficiency.html
12. Enzyme Regulation. Biology Energy and Enzymes. Khan Academy p. 2 Retrieved from https://www.khanacademy.org/science/biology/energy-and-enzymes/enzyme-regulation/a/enzyme-regulation
13. IBID – Lavrikova, pg. 5
14. Snyder, Kimberly. (December 06 2017) How Enzymes Affect Beauty and Health. Retrieved from https://www.huffingtonpost.com/kimberly-snyder/enzymes-beauty-health_b_1201847.html
15. IBID – Pugliese pg 391
16. IBID – Lavrikova p 7
17. Preland, R., Rothnagel, J., Lawrence, O. (2006)Chapter 9, Profilaggrin and the Fused S100 Family of Calcium-Binding Proteins. Skin Barrier, Elias & Feingold.
18. Ekholm, E., Brattasnd, M., Egelrud, T. (Jan. 2000) Stratum Corneum Tryptic Enzyme in Normal Epidermis: A Missing Link in the Desquamation Process? Journal of Investigative Dermatology, Vol 114, Issue 1, PP 56-63 Retrieved from https://www.sciencedirect.com/science/article/pii/S0022202X15407316
19. IBID – Pugliese – Ref 1
20. IBID – Ref 17, p. 56











