Abstract: The following review comprises background, literature and applications of biodegradable lamellar systems, their characteristics and limitations. The article is focussed on phosphatidylcholine containing preparations like liposomes, nanodispersions and derma membrane structures.
Introduction
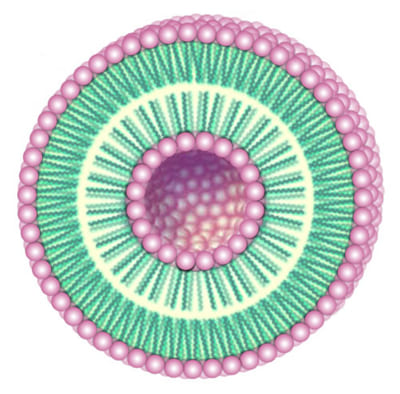 In the context of the revision of the European Cosmetic Directive, the particle size of carriers is being discussed. However in terms of the risk assessment of biodegradable liposomes and nanoparticles, no higher standards have been set since small intact particles will not merge into the human body even in the case of skin barrier disorders. That's why these systems which temporarily seemed to be old hat became attractive again overnight. The most important ingredient of these particles is the naturally occurring phosphatidylcholine.
In the context of the revision of the European Cosmetic Directive, the particle size of carriers is being discussed. However in terms of the risk assessment of biodegradable liposomes and nanoparticles, no higher standards have been set since small intact particles will not merge into the human body even in the case of skin barrier disorders. That's why these systems which temporarily seemed to be old hat became attractive again overnight. The most important ingredient of these particles is the naturally occurring phosphatidylcholine.
Publications and patents on liposomes and related nanodispersions, along with their different chemical components, preparation, and use in skincare products have often been reviewed. 1,2,3,4,5,6
The reviews do not need any additional comments. Of interest are general questions, such as why these lamellar particles should be used in cosmetics, what functionalities are expected from them, and what advantages they do provide compared with alternative formulations.
The relationship of the lamellar structures of liposomes and nanodispersions with lamellar creams based on phospholipids5, allows a smooth transition from an active agent dominated skin care7 to skin protection. As regards the skin care, the skin barrier can be opened for the penetration of active agents and then closed again without the risk of counterproductive occlusive conditions. This specific technique is rather significant for professional treatments.
Native phosphatidylcholine
Looking at the horny layer, whose structure9 protects the skin from external materials,phospholipids and phosphatidylcholine, in particular,play a minor role. The lipid bilayers contain only traces of phospholipids, and the main components are free fatty acids, cholesterol, triglycerides, hydrocarbons and ceramides.
But deeper in the living part of the epidermis, phosphatidylcholine is usually found as the most important constituent of all biological membranes, especially of plasma cell membranes. Over and above that, phosphatidylcholine is the source of phosphocholine to transform ceramides into sphingomyelins. In this context, phosphatidylcholine stands for living tissues, whereas the increase of ceramides in the cells means that their death by apoptosis is imminent.
Phosphatidylcholine of both human and vegetable origin shows a fatty acid composition, which is dominated by unsaturated fatty acids.
The fatty acid content of soy phosphatidylcholine, which is readily available and mostly used in cosmetic formulas, is characterized by a proportion of linoleic acid up to 70% of the total fatty acids. Consequently, soy phosphatidylcholine has a low phase-transition temperature of below 0 °C in water-containing systems. This is the reason behind its ability to fluidize the lipid bilayers of the horny layer, which can be determined by measuring the increase of the transepidermal water loss (TEWL) after application for a short while. The slight Increase of TEWL coincides with the penetration of hosphatidylcholine and active agents, which are co-formulated with phosphatidylcholine. Because of its high content of linoleic acid and penetration capability, soy phosphatidylcholine delivers linoleic acid very effectively into the skin, resulting in anti-acne properties10. By adhering very strongly to surfaces containing proteins like keratin, phosphatidylcholine shows conditioning and softening effects.
When native phosphatidylcholine is dispersed in water, it is practically inevitable that lamellar systems like liposomes will occur, because this is the most natural form of the material. For example, phosphatidylcholine swollen by water transforms spontaneously into liposomes when "disturbed" by little amounts of salts or watersoluble organic compounds like urea. In contrast,it has been known for a long time, that horny layer pre-treated by phosphatidylcholine can be penetrated much more easily by no encapsulated materials. So liposomes are not really needed to turn out the functionalities of phosphatidylcholine, but they are very convenient, because the handling of pure phosphatidylcholine requires a lot of experience and sometimes patience as well.
Phosphatidylcholine is known as a penetration enhancer; this property is usually associated with liposomes. Liposomes are the vesicles said to transport cosmetic agents better into the horny layer. That is true and, moreover, the conditioning effect causes the horny layer to become a depot for these agents. Measurements of systemically active pharmaceuticals revealed that an increase of enetration is not synonymous with an increase of permeation.
Actually, permeation of active agents is often slowed by phosphatidylcholine in such a way,that a high permeation peak in the beginning of the application is prevented. Instead, a more continuous permeation takes place outside the horny layer depot into the living part of the body over a longer period of time.
This property makes phosphatidylcholine and liposomes very attractive for the application of vitamins, provitamins, and other substances influencing the regenerating ability of the living epidermis. Hydrogenated phosphatidylcholine Liposomes consisting of unsaturated phosphatidylcholine do not strengthen the natural barrier function of the skin in the short term with the exception of its indirect effect of supporting the formation of ceramide I in the long term. Ceramide I is known for containing linoleic acid and for being one of the most important barrier-activating substances. Instead of unsaturated phosphatidylcholine, a fully saturated phosphatidylcholine should be selected for products designed for skin protection.
Saturated phosphatidylcholine stabilizes the normal TEWL similarly to ceramides when the horny layer is attacked by hydrophilic or lipophilic chemicals11,12,13,14. Saturated phosphatidylcholine is synonymous with hydrogenated soy phosphatidylcholine, which contains mainly stearic und palmitic acids. On the other side semi-synthetic compounds like dipalmitoylphosphatidylcholine (DPPC) and distearoylphosphatidylcholine (DSPC) are available.
The multifunctional properties of phosphatidylcholines lead to a number of different applications. So, formulations with unsaturated phosphatidylcholine are preferred to support skin regeneration, antiaging, acne preventing, and penetrating other active agents like vitamins and their derivatives into the skin. Formulations with hydrogenated phosphatidylcholine may be used for skin and sun protection. Because of their special properties it can make sense to combine unsaturated with saturated phosphatidylcholine in one and the same cosmetic or dermatological product.
Liposomes
Liposomes are spherical vesicles, whose membranes consist of one (unilamellar) or more (oligolamellar, multilamellar) bilayers of phosphatidylcholine. Sometimes, especially in patents, reference is made not about liposomes but about "vesicles with an internal aqueous phase". The vesicles can differ in size (diameter about 15-3500 nm) and shape (single and fused particles). At a given chemical composition, these parameters strongly depend on the process of preparation. Very often the preparations are metastable. That means the state of free enthalpy is not in equilibrium with the environment. As a result the vesicles change their lamellarity, size, size distribution, and shape with time. For example, small vesicles tend to form larger ones and large vesicles smaller ones. Fortunately this is mostly not critical for quality, because the properties of the phosphatidylcholine, which the vesicles are based on, remain unchanged as a rule. Nevertheless the stability seems to be the best in a range of about 100-300 nm. That is the case of pure aqueous dispersions of highly enriched (80-100%) soy phosphatidylcholine.
On and off it is discussed that multi-lamellar liposomes (multiple-shell, onion-shaped vesicles) can transport higher amounts of active agents than unilamellar ones (one-shell vesicles). This, however, rather seems to be a pseudo-discussion since cosmetic liposomes on the whole represent a potpourri of one-shell and multiple-shell vesicles which is due to the manufacturing process. The manufacturing of unilamellar liposomes would be far too expensive. On top of that, the number of shells (membranes) has no impact on the abovedescribed "transport mechanism". Solely the low or high dosage of the fluidizing membrane component (PC) influences the efficacy of liposomes.
In a complete formulation together with further ingredients, other influences like compatibility, concentration of salts, amphiphilics, and lipophilics play an important role. Therefore, it is often very difficult to prove the existence of liposomes, for example, in a gel phase15 or a creamy matrix. However, this is more a marketing problem than a problem of effectiveness of the formulation. Today, we can assume that the effectiveness of phosphatidylcholine is based more on the total chemical composition of the cosmetic product and less on the existence or non-existence of the added liposomes.
Of course, formulations are very effective in particular when consisting of pure liposomal dispersions bearing lipophilic additives in the membrane spheres and/or hydrophilics in the internal and external aqueous phases within the range of their bearing capacity. In this respect, there has been an intensive search to increase the encapsulation capacity of liposomes for lipids because consumers are used to applying lipid-rich formulations. Efforts were made to add emulsifier to the liposomal dispersions to stabilize higher amounts of lipids.
Formulators now know that the compatibility of liposomes with regard to emulsifiers is generally limited. In contrast, additional emulsifiers have a weakening effect on the barrier affinity of phosphatidylcholine. They cause the phosphatidylcholine and the lipids to be more easily removed from the skin while washing.
In this respect there is only one rational consideration:
- to make use of nanodispersions consisting of phosphatidylcholine and lipids instead of liposomes.
Liposomal dispersions have proved not only to be innovative and effective cosmetic ingredients, but also to be a very convenient form to work with phosphatidylcholine. In dermatology, they are used as such with success for preventing and treating several skin diseases.
Liposomal formulations may have a totally different functional quality. Just to mention an example: free vitamin C (ascorbic acid) in high concentrations has the same keratolytic effect as a fruit acid and hence can scale off the cells of the horny layer. Even so, it has little impact on the collagen synthesis due to the fact that the acid remains on the skin surface. However, if low concentrations of ascorbic acid esters of the phosphoric, stearic or palmitic acid are encapsulated into liposomes (water-soluble esters) or in biodegradable nanoparticles (fatsoluble esters), the ascorbic acid can be transported to the areas where it is actually needed. The esters are far more resistant to oxidation than free ascorbic acid. The carrier bodies have the size of about 50 to 200 nm. After the "transport" into the skin, the esters are enzymatically hydrolyzed into substances that are identical to the natural substances of the body and in the present case, into free ascorbic acid and phosphoric acid, stearic acid or palmitic acid. At this point, the ascorbic acid can take full effect.
Nanodispersions - biodegradable nanoparticles
Biodegradable nanoparticles5,16 are a consequence of the observation that oil droplets can fuse with liposomes, when the capacity of bilayers for lipids is exhausted17. Further increasing the lipid/phosphatidylcholine ratio and using high-pressure homogenizers lead to nanoparticles. Nanoparticles consist of emulsion- like oil droplets surrounded by a monolayer of phosphatidylcholine. The advantage of nanoparticles is that they allow formulations to tolerate more lipids and remain stable. Additional emulsifiers are not needed.
Further functions of nanoparticles are the protection of the encapsulated material against oxidation or against the impact of other substances,providing a convenient application of active agents, conditioning the skin and improving the bioavailability of active agents.
Conditioning the skin implies the reaction of the capsule material with the skin barrier. Like the phosphatidylcholine containing membranes of liposomes the membranes of nanoparticles analogously merge with the membranes of the skin barrier and make them more fluid and permeable. Thus the encapsulated active agents can so pass the skin barrier. The original membrane completely dissolves and the different ingredients slowly permeate into the skin in the form of molecules.
The increased dermal bioavailability of lipophilic active agents in nanodispersions allows reducing the concentration of lipophilic active agents. This not only is a very interesting economic aspect, it can also improve the tolerance and as far as pharmaceutical active agents are concerned, it can reduce the side effects too.
Liquid, biodegradable nanoparticles based on PC can be used as a medium for vitamin A, E and their esters (e.g. Tocopheryl Acetate and Retinyl Acetate). A wide range of applications are vegetable oils with their triglycerides whose acid components are long-chained and polyunsaturated (omega-3 and omega-6 acids).
These emulsifier-free dispersions can be applied like water, they are non-greasing, penetrate instantly and show a high anti-inflammatory potential due to the metabolites of essential fatty acids formed in the skin18. A typical field of application is the care of sun-damaged and atopic skin19.
Derma membrane structure
An interesting field of lamellar cosmetic compositions with hydrogenated soy phosphatidylcholine is the derma membrane structure (DMS20) technology 21. DMS stands for lamellar cream bases containing hydrogenated soy phosphatidylcholine, sebum-compatible medium chain triglycerides (MCT), phytosterols and squalane. In addition to liposomal dispersions and nanoparticles, DMS is a third way to formulate phosphatidylcholine with hydrophilic and lipophilic compounds free of further emulsifiers.
DMS is water- and sweat proof and therefore suitable for skin protection and sun creams without using silicones or mineral oil additives. It can easily be transformed into other final products by stirring at room temperature together with liquid lipids and/or hydrophilic active agents solved in water.
Lamellar cream bases are appropriate formulations for skin care but also for skin protection purposes due to their chemical composition and their physical characteristics22,23,24. Professional associations recommend the preparations for the occupational skin protection in particular for the contact with different working substances11 and for recovery purposes; meanwhile, skin protection and skin recovery are rated equally12.
The washout effect of lamellar cream bases in comparison to typical O/W emulsions is negligible with the result that the natural barrier structure with its characteristic conformation is largely maintained. This fact that is particularly important for problem skin. Hence they are appropriate formulations for the treatment of barrier disorders. As far as the supportive pre- vention is concerned, individual cosmetic formulations in strict accordance with the German Cosmetic Directive (KVO) can be prepared in the pharmacy. Regarding dermatological prescriptions25,26,27 the German Ordinance on the Operation of Pharmacies as to the definition of additives (cream bases) and pharmaceutical active agents has to be considered28,29. A number of active agents serve for dermatological but also for cosmetic preparations30; in these cases the respective regulation regarding the claims should be observed.
Topical treatments can either be realized on a modular base by preparing individual formulations or alternatively by applying finished lamellar products. In the foreground are preparations for the care of barrier, cornification and connective tissue disorders as well as sun protection preparations. The transition of the dermatological therapy to a cosmetic prevention can easily be realized.
Modular use of lamellar systems
As mentioned, DMS is predestined for skin protection, but by addition of nanoparticles and/or liposomal dispersions it can easily be enriched by unsaturated phosphatidylcholine containing esterified linoleic acid. The resulting products are creamy, stable, and anticomedogenic.
The effect of pure DMS basic creams on skin moisturizing, smoothing and tightening are still significant several days after finishing the application. A comparison of cosmetic and dermatological formulations based on medical indications shows that various skin disorders already can be cured by applying appropriate skin care preparations31,32,33. A characteristic is the release of active agents from lamellar preparations: liposomes with polar active agents, such as azelaic acid help avoid high initial dosages. Vitamin-A derivatives from phospholipidic nanodispersions show typical Vitamin-A acid effects already in low concentrations34.
Depot effects can be observed with cream bases which allow time-dependent dosage reductions35. The current state of knowledge on lamellar formulations has been reviewed36.
The improved permeability of the skin barrier based on the use of PC-containing liposomes and nanoparticles is particularly advantageous for the application of masks. If the permeability is to be reversed after the mask, a DMS cream can be applied.
Stability and limits of lamellar systems Like linoleic esters and linoleic glycerides, liposomal dispersions based on unsaturated phosphatidylcholine dispersions have to be stabilized by antioxidants. By thinking naturally, a complex of vitamins C and E can be used with success. In some cases phosphatidylcholine and urea seem to stabilize each other37,38. Moreover, agents that are able to mask traces of radical-forming ions of heavy metals, like iron, can be added. Such additives are chelators like citrates, phosphonates, or ethylene diamine tetraacetic acid (EDTA). Alternatively, the unsaturated phosphatidylcholine can be substituted by a saturated one like DPPC or hydrogenated soy phosphatidylcholine, which should be favoured for its price. Because of the higher phase-transition temperature, liposomal dispersions based on hydrogenated material are more sophisticated in their preparation and are reserved for pharmacological applications as a rule.
Liposomes, nanoparticles and DMS have to be microbiologically stabilized. This may be a problem, because phosphatidylcholine like lecithin inactivates most of the conventional preservatives39.
In contrast, preservatives should not be penetrated in the skin to prevent irritation and sensitization. Therefore, glycols like propyleneglycol, glycerol, butyleneglycol, pentyleneglycol, hexyleneglycol, sorbitol and their mixtures are the compounds of choice. In contrast to ethanol, which can also be applied up to certain extent, these polyols show a moisturizing effect at the same time.
One of the reasons to substitute phosphatidylcholine by polyglycerols and other synthetic derivatives at the beginning of the liposomal developments was its hydrolytic instability in aqueous preparations for longer periods of time and at higher temperatures. In fact phosphatidylcholine, like other glycerides, is attacked by water to form lysophosphatidylcholine and free fatty acids. But the cleavage of the glyceride bond occurs mainly at a pH >7; so formulations in the range of pH 5,5-7 are sufficiently stable for most purposes. It is possible that hydrolysis depends on the amount of additional surface active compounds. This is another reason to use liposomal dispersions without additional emulsifiers.
Liposomes and nanoparticles based on PC are incompatible with a series of substances commonly used in cosmetic preparations. They are sensitive to emulsifiers, tensides and solvents above all ñ like the skin after dissolving the lamellar structures.
As far as encapsulating is concerned, the molecular weight of the active agents is of significance. Macromolecules like hyaluronic acid, polysaccharides and proteins only form physical mixtures with PC-containing carriers. These physical mixtures however also are beneficial due to the above-described reactions of the carriers. With regard to skin hydration and skin smoothing for instance they can complement each other very well.
In addition, it has been observed that added, non-encapsulated low molecular substances also benefit from the fluidization of the skin barrier and that their dermal bioavailability increases. Even highly polar substances such as amino acids, azelaic acid, fumaric acid, caffeine as well as hydrophilic vegetable extracts such as green tea, eyebright or butcher's broom can pass through the skin barrier.
Conclusion
Generally, members of the lamellar family like liposomes, nanoparticles and DMS are more compatible with the skin structure than usually applied conventional emulsions. "Compatible" means that formulations are physiologic, do not disturb the integrity of the skin lipid bilayers and are not washed out when the skin is cleansed. In the sense of modern strategies of cosmetics, these formulations get by with a minimum of auxiliary compounds, which put only a strain on the skin. Moreover, compatibility means embedding lipids and hydrophilic agents in the horny layer and being in line with the natural situation. Remarkably, phosphatidylcholine need not be applied in high concentrations, because experience shows that formulations are physically stable at lower amounts. Also, there is a cumulative effect in the horny layer with repeated application of phosphatidylcholine. In many cases liposomes, nanoparticles and DMS are compatible with each other in a sense that they can be used as a modular system.
References:
1 Lasic DD, Liposomes and niosomes. In: Rieger MM, Rhein LD, eds. Surfactants in Cosmetics, 2nd edition New York: Marcel Dekker, 1997:263-283
2 Wendel A, Lecithins, phospholipids, liposomes in cosmetics, dermatology and in washing and cleansing preparations, Verlag für chemische Industrie, Augsburg 1994
3 Wendel A, Lecithins, phospholipids, liposomes in cosmetics, dermatology and in washing and cleansing preparations Part II, Verlag für chemische Industrie, Augsburg 1997
4 Braun-Falco O, Korting HC, Maibach HI, eds. Liposome Dermatics, Springer-Verlag, Berlin 1992
5 Lautenschläger H, Handbook of Cosmetic Science and Technology edited by Barel AO, Paye M and Maibach HI, CRC Press Taylor & Francis Group, Boca Raton 2006:155-163
6 Lautenschläger H, Huckepack – Übersicht Trägersysteme, medical Beauty Forum 2013;1:16-18
7 Lautenschläger H, Albrecht M, Bohn M, Weisser M, Hautschutzpräparate zur Prävention von Hautschäden, DE 19857490 (14.12.98)
8 Lautenschläger H, Angewandte Korneotherapie in der Hautpflege – ein Leitfaden für die Anti-Aging-Behandlung, Ästhetische Dermatologie (mdm) 2007;3:8-16
9 Iwai I et al, The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide shingoid moiety, J Invest Dermatol 2012 Sep;132(9):2215-25
10 Ghyczy M, Nissen H-P, Biltz H, The treatment of acne vulgaris by phosphatidylcholine from soybeans, with a high content of linoleic acid, J Appl Cosmetol 1996;14:137-145
11 Lautenschläger H, Kühlschmierstoffe und Hautschutz - neue Perspektiven, Mineralöltechnik 1998;5:1-16
12 Lautenschläger H, Kühlschmierstoffe – Forderungen des modernen Hautschutzes, Mineralöltechnik 1996;6:1-19
13 Lautenschläger H, Hautschutz für Hände starker Männer, Pharmazeutische Zeitung 1999:144(13):1038-1040
14 H. Lautenschläger, Ein neues Konzept im betrieblichen Hautschutz, Jahrbuch für den Praktiker, Augsburg, 1998:121-126
15 Müller T, Röding J, Lautenschläger H, Elektronenmikroskopischer Nachweis von Liposomen in einem Hautpflegegel, SÖFW 1989;115(3):88-89
16 Lautenschläger H, Nanopartikel in Kosmetika – gut oder schlecht?, Beauty Forum 2009;5:44-47
17 Lautenschläger H, Liposomes in dermatological preparations Part II, Cosmetics & Toiletries 1990;105 (7):63-72
18 Lautenschläger H, Das ABC der Fettsäuren, Beauty Forum 2009;12:40-47 19 Lautenschläger H, Lichtgeschädigte Haut: Sonnenbad – das hilft danach, Kosmetische Praxis 2006;3:8-9
20 Trade mark of Kuhs GmbH, Lörrach
21 Kutz G, Galenische Charakterisierung ausgewählter Hautpflegeprodukte, Pharmazeutische Zeitung 1997; 142 (45):4015-4019
22 Lautenschläger H, Albrecht M, Bohn M, Weisser M, Wasserhaltige Hautschutzpräparate zur Prävention von Hautschäden, DE 19857492 (14.12.98)
23 Lautenschläger H, Universelle Basiscremes mit Membran-Struktur für Hautpflege, Hautschutz und Dermatika, Österreichische Apothekerzeitung 2002;56;14:679
24 Derma Membran Struktur®: Fortschritt im betrieblichen Hautschutz, Symposium Medical 2001;12;5:37
25 Valenta C, Salbengrundlagen; ÖAZ 2005;16:770-773
26 Valenta C, Stabilität: Cyproteronacetat in magistralen Zubereitungen, ÖAZ 2002;56:676- 678 27 Wolf G, Höger PH, Dermatologische Basistherapie mit hypoallergenen und noxenfreien Externa im Kindesalter, JDDG 2009;7:50-60
28 Apothekenbetriebsordnung, Pharmazeutische Zeitung 2012;12;Supplement:1-54
29 Zipp S, Wareneingangskontrolle von Wirkund Hilfsstoffen mit dem Fokus auf GMP und regulatorische Anforderungen, Pharm. Ind. 2012;74;4:547-555
30 Lautenschläger H, Synergien nutzen – Wie Wirkstoffe und Cremebasen Kosmetik und Pharmazie verbinden, osmetische Praxis 2010;3:10-12
31 Lautenschläger H, Übersicht: Behandlung von Problemhäuten, Kosmetik International 2012;8:16-18
32 Lautenschläger H, Grenzgänger – Kosmetische Pflege auf den Punkt gebracht, Beauty Forum 2010;8:27-29
33 Lautenschläger H, Gegenüberstellung – kosmetische und pharmazeutische Wirkstoffe, Kosmetik International 2010;10:32-36
34 Lautenschläger H, Vitamine in der Kosmetik, medical Beauty Forum 2011;1:14-16 and 2001;2:16-18
35 Lautenschläger H, Nervensache – erwünschte und unerwünschte Effekte, Kosmetik International 2013;2:40-42
36 Lautenschläger H, Korneotherapie – Bindeglied zwischen Dermatologie und Kosmetik (ISBN 978-3-00-035755-8), 2011:269-270
37 Nippon Surfactant Kogyo KK, Japanese Patent 199104364104 (1992)
38 Lautenschläger H, Hautbehandlungsmittel mit hohen Lipidgehalten unter Verwendung eines Bilayer enthaltenden Systems, Salzen organischer Säuren, Alkohol und Stabilisator, DE 4021082 (3.7.90)
39 Wallhäusser KH, Praxis der Sterilisation, Desinfektion – Konservierung. 5th ed. Stuttgart: Georg Thieme Verlag, 1995:43, 394











